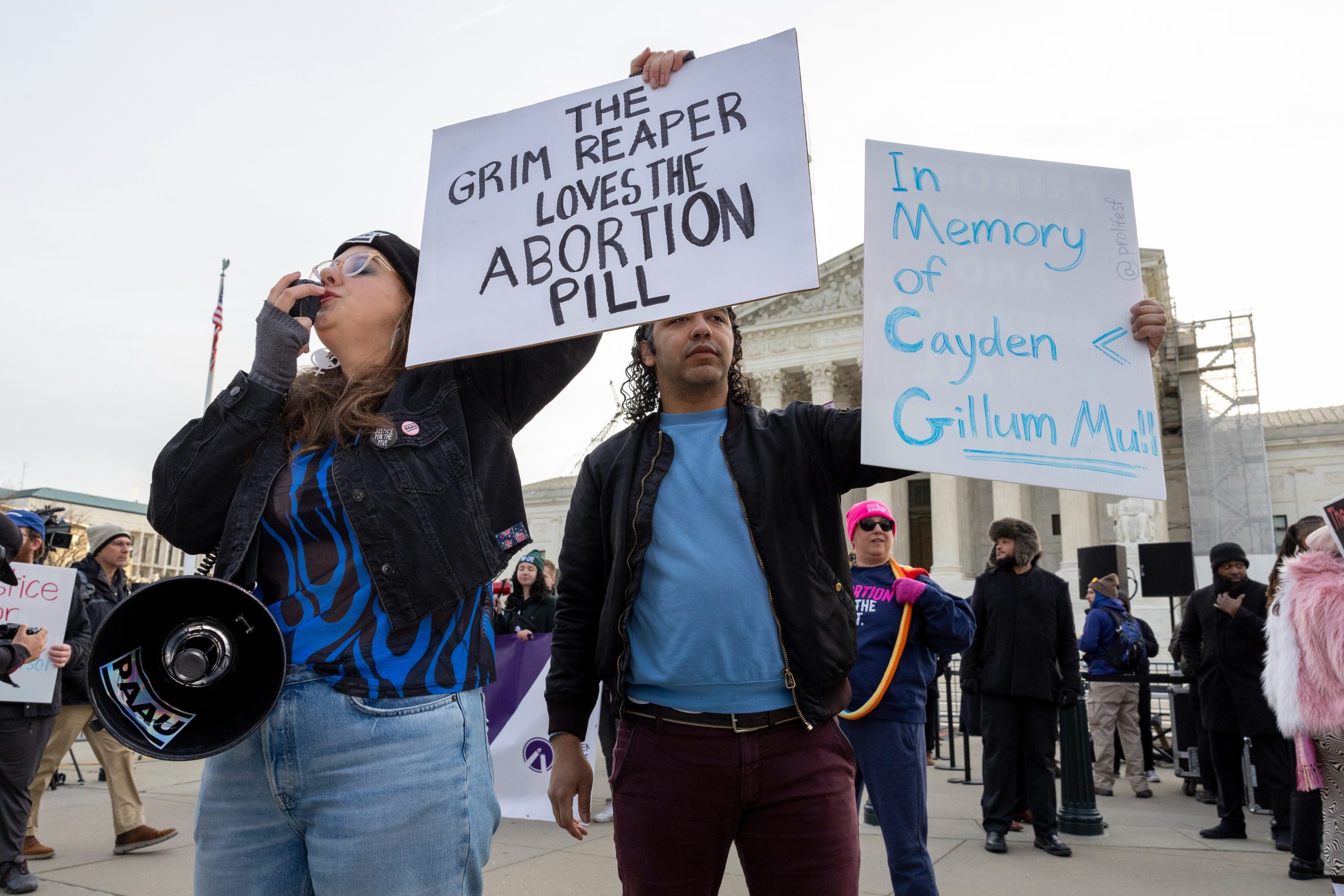The U.S. Supreme Court recently heard arguments that seem to point towards maintaining access to mifepristone, a key medication used in a majority of abortions in the United States.
During the session, a clear consensus emerged among the justices that the groups opposing the drug’s FDA approval and its easier access conditions may not have the legal standing to sue.
This outcome could keep the current regulations intact, allowing the medication to be received through mail and used for abortions up to 10 weeks of pregnancy.
This development occurs amidst significant shifts in abortion regulations following the Supreme Court’s 2022 decision, resulting in strict abortion bans in multiple states governed by Republicans.
Abortion medication Protest (Credits: The Columbian)
The court’s discussion focused on whether anti-abortion doctors and organizations have the right to challenge the FDA’s actions. Even Justices Amy Coney Barrett, Neil Gorsuch, and Brett Kavanaugh, who previously supported overturning Roe v. Wade, seemed skeptical of the plaintiffs’ standing.
The heart of the debate is whether to uphold a decision by a conservative appeals court that would restrict access to mifepristone. This case’s implications could reach beyond the courtroom, potentially influencing future political races.
Another related legal battle looms, with the court expected to decide on the necessity of providing abortions in emergencies at hospitals, even in states with abortion bans. A victory for the opponents of mifepristone could disrupt its delivery through mail and large pharmacies and halt telehealth services prescribing the drug.
The Biden administration and the drug’s manufacturers have cautioned that such a ruling might challenge the FDA’s authority on drug approval and question its scientific determinations. They argue that mifepristone is among the safest medications approved by the FDA.
Justice Ketanji Brown Jackson, who joined the court after its last major abortion decision, appeared to lean towards arguments supporting the current FDA regulations when questioning the role of judges in reviewing medical studies. Opponents of the drug claim that the FDA’s loosening of restrictions in recent years compromises women’s health.
US Supreme Court ruling on abortion pill (Credits: France 24)
The case, initiated after the overturning of Roe v. Wade, has seen a mixed legal journey. It began with a broad ruling from a Texas judge that could have entirely stripped mifepristone of its approval. Still, an appeals court narrowed this decision, targeting only the FDA’s recent relaxations on the drug’s distribution.
The Supreme Court’s choice to review this matter has focused on technicalities, such as the Comstock Act, which opponents have cited in their argument against mail delivery of mifepristone. Mifepristone, used alongside misoprostol for medication abortions, has been a choice for over 6 million people since its introduction in 2000.
If access to mifepristone is limited, healthcare providers might have to rely solely on misoprostol, which doesn’t end pregnancies as effectively. This case underlines the ongoing legal and societal debates over abortion access and the role of regulatory bodies in healthcare decisions.